
HL Paper 2
Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
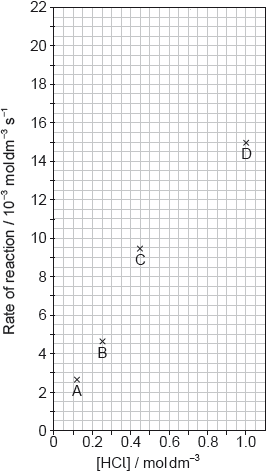
The results of a series of experiments in which the concentration of HCl was varied are shown below.
Outline two ways in which the progress of the reaction can be monitored. No practical details are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Draw the best fit line for the reaction excluding point D.
Suggest the relationship that points A, B and C show between the concentration of the acid and the rate of reaction.
Deduce the rate expression for the reaction.
Calculate the rate constant of the reaction, stating its units.
Predict from your line of best fit the rate of reaction when the concentration of HCl is 1.00 mol dm−3.
Describe how the activation energy of this reaction could be determined.
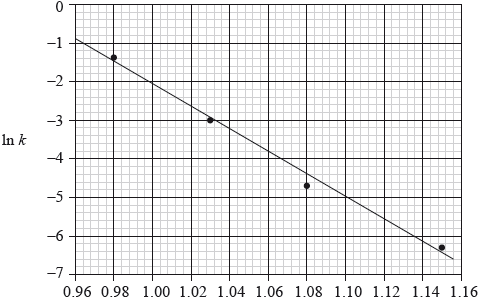
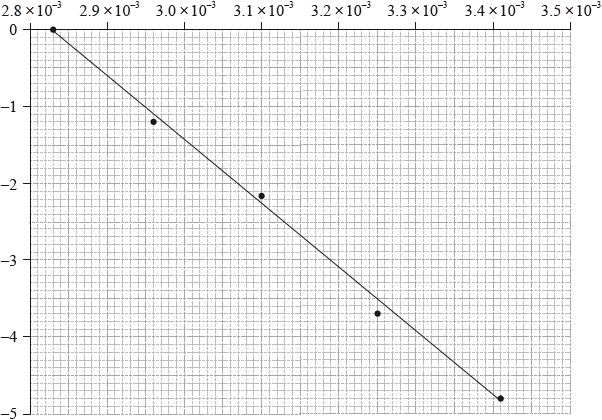
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\) (temperature in Kelvin) for the second order decomposition of \({{\text{N}}_{\text{2}}}{\text{O}}\) into \({{\text{N}}_{\text{2}}}\) and O.
\[{{\text{N}}_{\text{2}}}{\text{O}} \to {{\text{N}}_{\text{2}}} + {\text{O}}\]
\[\frac{1}{T}/{10^{ - 3}}{\text{ }}{{\text{K}}^{ - 1}}\]
State how the rate constant, \(k\) varies with temperature,\(T\).
Determine the activation energy, \({E_{\text{a}}}\), for this reaction.
The rate expression for this reaction is rate \( = k{{\text{[}}{{\text{N}}_{\text{2}}}{\text{O]}}^{\text{2}}}\) and the rate constant is \({\text{0.244 d}}{{\text{m}}^{\text{3}}}{\text{mo}}{{\text{l}}^{ - 1}}{{\text{s}}^{ - 1}}\) at 750 °C.
A sample of \({{\text{N}}_{\text{2}}}{\text{O}}\) of concentration \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) is allowed to decompose. Calculate the rate when 10% of the \({{\text{N}}_{\text{2}}}{\text{O}}\) has reacted.
To determine the activation energy of a reaction, the rate of reaction was measured at different temperatures. The rate constant, \(k\), was determined and \(\ln k\) was plotted against the inverse of the temperature in Kelvin, \({T^{ - 1}}\). The following graph was obtained.
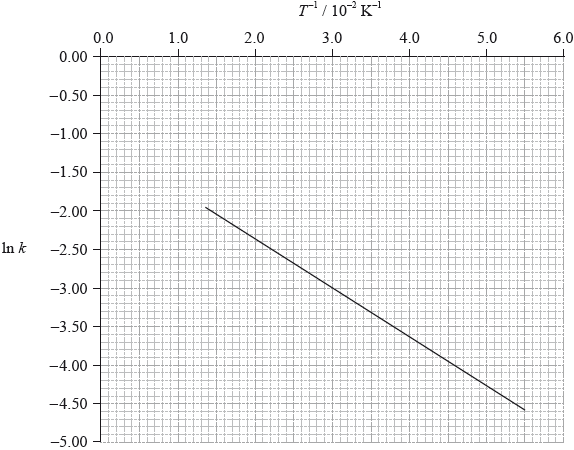
Define the term activation energy, \({E_{\text{a}}}\).
Use the graph on page 8 to determine the value of the activation energy, \({E_{\text{a}}}\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
On the graph on page 8, sketch the line you would expect if a catalyst is added to the reactants.
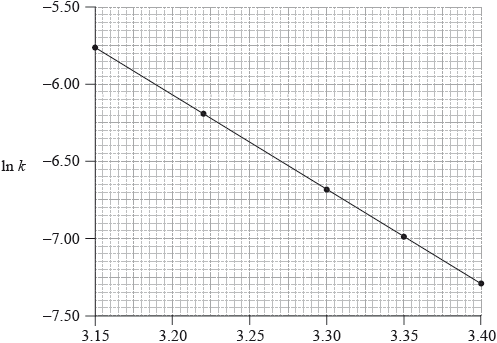
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\).
\[\frac{1}{T}/{10^{ - 3}}{\text{ }}{{\text{K}}^{ - 1}}\]
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\). Define the term activation energy, \({E_{\text{a}}}\).
State how the rate constant, k , varies with temperature, T.
Determine the activation energy, \({E_{\text{a}}}\), correct to three significant figures and state its units.
Reaction kinetics can be investigated using the iodine clock reaction. The equations for two reactions that occur are given below.
Reaction A: \({{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Reaction B: \({\text{ }}{{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}\)
Reaction B is much faster than reaction A, so the iodine, \({\text{I}_2}\), formed in reaction A immediately reacts with thiosulfate ions, \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\), in reaction B, before it can react with starch to form the familiar blue-black, starch-iodine complex.
In one experiment the reaction mixture contained:
5.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 2.00 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrogen peroxide (\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\))
5.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 1% aqueous starch
20.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 1.00 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid (\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\))
20.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 0.0100 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium thiosulfate (\({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\))
50.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of water with 0.0200 ± 0.0001 g of potassium iodide (KI) dissolved in it.
After 45 seconds this mixture suddenly changed from colourless to blue-black.
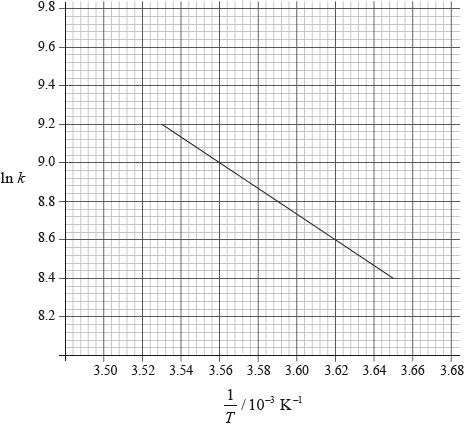
The activation energy can be determined using the Arrhenius equation, which is given in Table 1 of the Data Booklet. The experiment was carried out at five different temperatures. An incomplete graph to determine the activation energy of the reaction, based on these results, is shown below.
The concentration of iodide ions, \({{\text{I}}^ - }\), is assumed to be constant. Outline why this is a valid assumption.
For this mixture the concentration of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), can also be assumed to be constant. Explain why this is a valid assumption.
Explain why the solution suddenly changes colour.
Calculate the total uncertainty, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of the volume of the reaction mixture.
Calculate the percentage uncertainty of the concentration of potassium iodide solution added to the overall reaction mixture.
Determine the percentage uncertainty in the concentration of potassium iodide in the final reaction solution.
The colour change occurs when \(1.00 \times {10^{ - 4}}{\text{ mol}}\) of iodine has been formed. Use the total volume of the solution and the time taken, to calculate the rate of the reaction, including appropriate units.
State the labels for each axis.
x-axis:
y-axis:
Use the graph to determine the activation energy of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), correct to three significant figures.
In another experiment, 0.100 g of a black powder was also added while all other concentrations and volumes remained unchanged. The time taken for the solution to change colour was now 20 seconds. Outline why you think the colour change occurred more rapidly and how you could confirm your hypothesis.
Consider the following reaction studied at 263 K.
\[{\text{2NO(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2NOCl(g)}}\]
It was found that the forward reaction is first order with respect to \({\rm{C}}{{\rm{l}}_2}\) and second order with respect to NO. The reverse reaction is second order with respect to NOCl.
Consider the following equilibrium reaction.
\[\begin{array}{*{20}{c}} {{\text{C}}{{\text{l}}_2}({\text{g)}} + {\text{S}}{{\text{O}}_2}({\text{g)}} \rightleftharpoons {\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}({\text{g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
In a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container, at 375 °C, \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}\) and \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{C}}{{\text{l}}_{\text{2}}}\) were introduced. At equilibrium, \({\text{7.65}} \times {\text{1}}{{\text{0}}^{ - 4}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) was formed.
State the rate expression for the forward reaction.
Predict the effect on the rate of the forward reaction and on the rate constant if the concentration of NO is halved.
1.0 mol of \({\rm{C}}{{\rm{l}}_2}\) and 1.0 mol of NO are mixed in a closed container at constant temperature. Sketch a graph to show how the concentration of NO and NOCl change with time until after equilibrium has been reached. Identify the point on the graph where equilibrium is established.
Consider the following reaction.
\[{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}} \to {\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\]
Possible reaction mechanisms are:
\(\begin{array}{*{20}{l}} {{\text{Above 775 K:}}}&{{\text{N}}{{\text{O}}_2} + {\text{CO}} \to {\text{NO}} + {\text{C}}{{\text{O}}_{\text{2}}}}&{{\text{slow}}} \\ {{\text{Below 775 K:}}}&{{\text{2N}}{{\text{O}}_2} \to {\text{NO}} + {\text{N}}{{\text{O}}_{\text{3}}}}&{{\text{slow}}} \\ {}&{{\text{N}}{{\text{O}}_3} + {\text{CO}} \to {\text{N}}{{\text{O}}_2} + {\text{C}}{{\text{O}}_2}}&{{\text{fast}}} \end{array}\)
Based on the mechanisms, deduce the rate expressions above and below 775 K.
State two situations when the rate of a chemical reaction is equal to the rate constant.
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\) for the first order decomposition of \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\) into \({\text{N}}{{\text{O}}_{\text{2}}}\). Determine the activation energy in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for this reaction.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Determine the value of the equilibrium constant, \({K_{\text{c}}}\).
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
Hydrogen peroxide decomposes according to the equation below.
\({\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\)
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
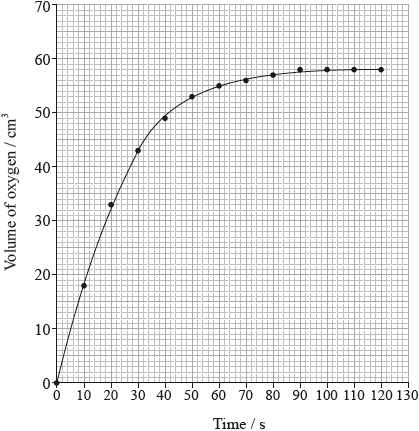
Outline how the initial rate of reaction can be found from the graph.
Explain how and why the rate of reaction changes with time.
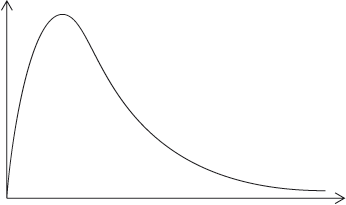
A Maxwell-Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
(i) In some reactions, increasing the concentration of a reactant does not increase the rate of reaction. Describe how this may occur.
(ii) Consider the reaction
\[{\text{2A}} + {\text{B}} \to {\text{C}} + {\text{D}}\]
The reaction is first order with respect to A, and zero order with respect to B. Deduce the rate expression for this reaction.
Sketch a graph of rate constant \((k)\) versus temperature.
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = - 57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(i) Define standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Zinc is found in the d-block of the periodic table. Explain why it is not considered a transition metal.
(ii) Explain why \({\text{F}}{{\text{e}}^{3 + }}\) is a more stable ion than \({\text{F}}{{\text{e}}^{2 + }}\) by reference to their electron configurations.
A student used a pH meter to measure the pH of different samples of water at 298 K.
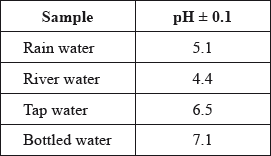
Use the data in the table to identify the most acidic water sample.
Calculate the percentage uncertainty in the measured pH of the rain water sample.
Determine the ratio of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) in bottled water to that in rain water.
\[\frac{{[{H^ + }]{\text{ }}in{\text{ }}bottled{\text{ }}water}}{{[{H^ + }]{\text{ }}in{\text{ }}rain{\text{ }}water}}\]
Determine the concentration of hydroxide ions in the sample of river water.
The acidity of non-polluted rain water is caused by dissolved carbon dioxide. State an equation for the reaction of carbon dioxide with water.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
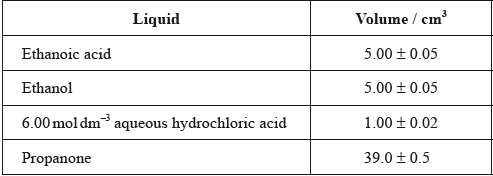
One group made the following initial mixture:
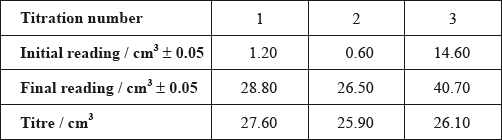
After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:
The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The concentration of ethanoic acid can be calculated as \({\text{1.748 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the percentage uncertainty of this value. (Neglect any uncertainty in the density and the molar mass.)
Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^3}\)).
Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
\({\text{3.00 c}}{{\text{m}}^{\text{3}}}\) of the \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the hydrochloric acid present in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the concentration of ethanoic acid in the final equilibrium mixture.
Deduce the equilibrium constant expression for the reaction.
The other concentrations in the equilibrium mixture were calculated as follows:
Use these data, along with your answer to part (iii), to determine the value of the equilibrium constant. (If you did not obtain an answer to part (iii), assume the concentrations of ethanol and ethanoic acid are equal, although this is not the case.)
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromomethane reacts with aqueous sodium hydroxide. State the organic product of this reaction.
Explain why the rate of the reaction between iodomethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}\), and NaOH(aq) is faster than the rate of the reaction between \({\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}}\) and NaOH(aq).
Bromine can be produced by the electrolysis of molten sodium bromide.
Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Predict the products formed at the electrodes during the electrolysis of concentrated aqueous sodium bromide.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide.
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Define the term standard electrode potential, \({E^\Theta }\).
Draw a labelled diagram for the voltaic cell in which the following reaction occurs.
\[{\text{Mg(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\]
Include in your answer the direction of electron flow and the polarity of the electrodes.
A student measures a voltage of 2.65 V in the voltaic cell formed between magnesium and copper half-cells using a digital voltmeter.
State the random uncertainty of this value, in V, and the number of significant figures in the answer.
Random uncertainty:
Significant figures:
Outline how the student can reduce the random error in her results.
Determine the standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of NaCl(s), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using a Born-Haber cycle and tables 7, 10 and 13 of the data booklet. The standard enthalpy change of atomization (standard enthalpy change of sublimation), \(\Delta H_{{\text{at}}}^\Theta \), of Na(s) is \( + {\text{108 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
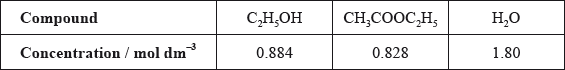
A graph of the successive ionization energies of magnesium is shown below.
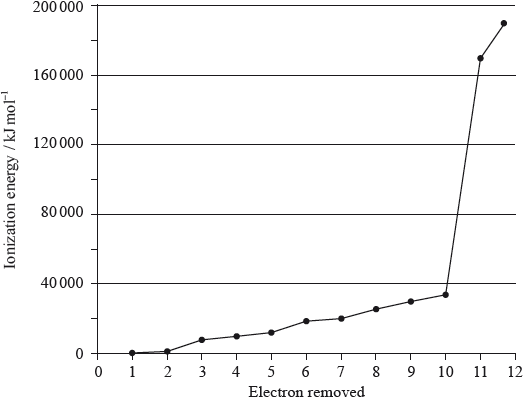
The graph below shows pressure and volume data collected for a sample of carbon dioxide gas at 330 K.
(i) Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
(ii) Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
(i) Explain the increase in ionization energy values from the 3rd to the 8th electrons.
(ii) Explain the sharp increase in ionization energy values between the 10th and 11th electrons.
(i) Magnesium reacts with oxygen to form an ionic compound, magnesium oxide. Describe how the ions are formed, and the structure and bonding in magnesium oxide.
(ii) Carbon reacts with oxygen to form a covalent compound, carbon dioxide. Describe what is meant by a covalent bond.
(iii) State why magnesium and oxygen form an ionic compound while carbon and oxygen form a covalent compound.
(i) Predict the type of hybridization of the carbon and oxygen atoms in \({\text{C}}{{\text{O}}_{\text{2}}}\).
(ii) Sketch the orbitals of an oxygen atom in \({\text{C}}{{\text{O}}_{\text{2}}}\) on the energy level diagram provided, including the electrons that occupy each orbital.

(iii) Define the term electronegativity.
(iv) Explain why oxygen has a larger electronegativity than carbon.
(i) Draw a best-fit curve for the data on the graph.
(ii) Use the data point labelled X to determine the amount, in mol, of carbon dioxide gas in the sample.
(i) Most indicators are weak acids. Describe qualitatively how indicators work.
(ii) Identify a suitable indicator for a titration between a weak acid and a strong base, using Table 16 of the Data Booklet.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
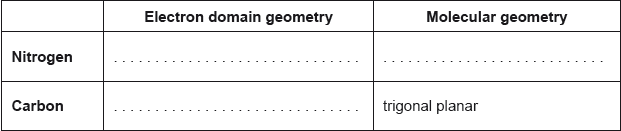
The structural formula of urea is shown.
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
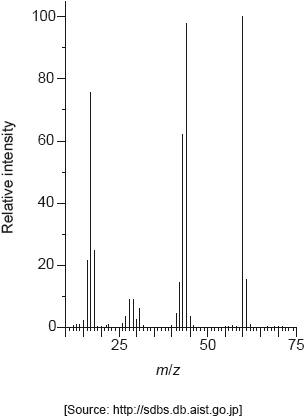
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
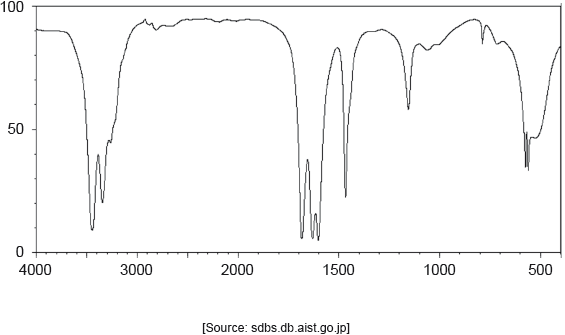
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
A student decided to determine the molecular mass of a solid monoprotic acid, HA, by titrating a solution of a known mass of the acid.
The following recordings were made.
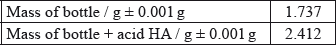
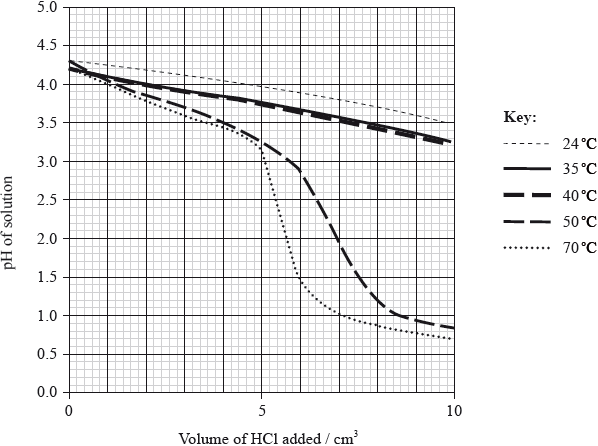
To investigate the effect of temperature on the effectiveness of a buffer solution, the student placed \({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of the buffer solution in a water bath at 24 °C. He added small portions of hydrochloric acid, stirring after each addition, until a total of \({\text{10 c}}{{\text{m}}^{\text{3}}}\) was added, and measured the pH continuously during the addition. The procedure was repeated at different temperatures and the results are shown in the following graph.
Determine the molecular formula of HA.
State what is meant by a buffer solution.
With reference to the graph on page 4, describe the effect of increasing temperature on the effectiveness of the buffer solution.
Ethanol has many industrial uses.
State an equation for the formation of ethanol from ethene and the necessary reaction conditions.
Equation:
Conditions:
Define the term average bond enthalpy.
Ethanol can be used as a fuel. Determine the enthalpy of combustion of ethanol at 298 K, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), using the values in table 10 of the data booklet, assuming all reactants and products are gaseous.
Students can also measure the enthalpy of combustion of ethanol in the laboratory using calorimetry. Suggest the major source of systematic error in these procedures.
State the equation for the acid-catalysed reaction of ethanol with propanoic acid and state the name of the organic product.
Equation:
Name of the organic product:
A polyester can be formed when ethane-1,2-diol reacts with benzene-1,4-dicarboxylic acid.
Deduce the structure of the repeating unit and state the other product formed.
Repeating unit:
Other product:
State the type of polymerization that occurs.
The standard enthalpy change of combustion, \(\Delta H_{\text{c}}^\Theta \), of propanoic acid is \( - 1527{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine the standard enthalpy change of formation of propanoic acid, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using this information and data from table 12 of the data booklet.
Deduce, giving a reason, the sign of the standard entropy change of the system for the formation of propanoic acid from its elements.
Identify three allotropes of carbon and describe their structures.
This question is about carbon and chlorine compounds.
Ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
Chloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{Cl}}\), can undergo polymerization. Draw a section of the polymer with three repeating units.
Compound A and compound B are hydrocarbons.
(i) State the term that is used to describe molecules that are related to each other in the same way as compound A and compound B.
(ii) Suggest a chemical test to distinguish between compound A and compound B, giving the observation you would expect for each.
Test:
Observation with A:
Observation with B:
Outline how you could use the IR spectra of compounds A and B and section 26 of the data booklet to identify them.
Two signals occur in the 1H NMR spectrum of compound A. Deduce their expected chemical shift and their splitting pattern, using section 27 of the data booklet.
A compound with a molecular formula C7H14O produced the following high resolution 1H NMR spectrum.
Deduce what information can be obtained from the 1H NMR spectrum.
Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of this compound using section 26 of the data booklet and the 1H NMR.
Suggest the structural formula of this compound.
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
Deduce the structural formula of the main organic product when hex-1-ene reacts with hydrogen bromide.
State the reagents and the name of the mechanism for the nitration of benzene.
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
A student titrated two acids, hydrochloric acid, HCl (aq) and ethanoic acid, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine their concentration. The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of each acid.
Using the graph, estimate the initial temperature of the solutions.
Determine the maximum temperature reached in each experiment by analysing the graph.
Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) At exactly 600°C the value of the equilibrium constant is 0.200. Calculate the standard Gibbs free energy change, , for the reaction, in kJ, using sections 1 and 2 of the data booklet. State your answer to three significant figures.
(iii) The standard enthalpy change of formation of phosgene, \(\Delta H_f^\Theta \), is −220.1kJmol−1. Determine the standard enthalpy change, \(\Delta H_{}^\Theta \), for the forward reaction of the equilibrium, in kJ, using section 12 of the data booklet.
(iv) Calculate the standard entropy change, \(\Delta S_{}^\Theta \), in JK−1, for the forward reaction at 25°C, using your answers to (a) (ii) and (a) (iii). (If you did not obtain an answer to (a) (ii) and/or (a) (iii) use values of +20.0 kJ and −120.0 kJ respectively, although these are not the correct answers.)
One important industrial use of phosgene is the production of polyurethanes. Phosgene is reacted with diamine X, derived from phenylamine.
(i) Classify diamine X as a primary, secondary or tertiary amine.
(ii) Phenylamine, C6H5NH2, is produced by the reduction of nitrobenzene, C6H5NO2. Suggest how this conversion can be carried out.
(iii) Nitrobenzene can be obtained by nitrating benzene using a mixture of concentrated nitric and sulfuric acids. Formulate the equation for the equilibrium established when these two acids are mixed.
(iv) Deduce the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
The other monomer used in the production of polyurethane is compound Z shown below.
(i) State the name, applying IUPAC rules, of compound Z and the class of compounds to which it belongs.
Name:
Class:
(ii) Deduce the number of signals you would expect to find in the 1H NMR spectrum of compound Z, giving your reasons.
The mass spectrum and infrared (IR) spectrum of compound Z are shown below:
Mass spectrum
IR spectrum
(iii) Identify the species causing the large peak at m/z=31 in the mass spectrum.
(iv) Identify the bond that produces the peak labelled Q on the IR spectrum, using section 26 of the data booklet.
Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of phenylamine at 298K using section 21 of the data booklet.